Introduction of New Vaccines for Immunization in Pregnancy - Programmatic, Regulatory, Safety and Ethical Considerations
Global Healthcare Consulting (Kochhar); Erasmus MC, University Medical Center (Kochhar); University of Washington (Kochhar, Ortiz); Vanderbilt University Medical Center (Edwards); Pan American Health Organization, or PAHO (Alvarez); Centers for Disease Control and Prevention, or CDC (Moro); University of Maryland School of Medicine (Ortiz)
"[C]ommunication, advocacy, and social mobilization are useful to increase awareness among key stakeholders about the importance of immunization of pregnant women, ... avoiding miscommunication and rumors..."
Vaccines against tetanus, pertussis, and seasonal influenza have been recommended for routine immunisation in pregnant women in high-income countries (HICs) and in some low and middle- income countries (LMICs) for many years. In spite of this, their uptake has been variable. Meanwhile, new vaccines for administration to pregnant women are currently under development, such as respiratory syncytial virus (RSV), group B streptococcus (GBS), cytomegalovirus (CMV), and monovalent pertussis vaccines. This article looks at some of the challenges associated with implementing an immunisation in pregnancy programme for a new vaccine, presenting a framework to help guide efforts in this area.
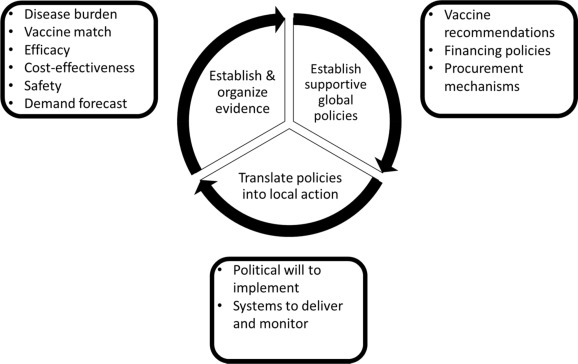
The schematic presented in the paper organises the major steps in the process of establishing and organising evidence, developing supportive global policies, and translating policies into local action. International and national coordination efforts, proactive planning from conception to implementation, and country-specific, cultural, and local factors that must be considered during the implementation of the immunisation in pregnancy programmes are outlined.
Factors that have communication-related components include:
- Coordination among the stakeholders and regional platforms for immunisation in pregnancy (e.g., vaccine manufacturers, national regulatory bodies, ethics committees, National Immunization Technical Advisory Groups (NITAGs), pharmacovigilance programmes, maternal and child health and immunisation programmes, funders, healthcare workers (HCWs), scientific communities, professional societies, non-governmental organisations (NGOs), religious and community leaders, pregnant women, and the media);
- Consistent tools, documents (forms, reports) and information technology (IT) platforms across different programmes and services;
- Communication plans for the public and key stakeholders; and
- Crisis plans for thorough and timely response to adverse events and communication to the public and media.
Communication-related challenges include:
- Lack of integrated approaches among stakeholders and the different programmes;
- Lack of promotion of maternal immunisation policies by health authorities;
- Incorrect beliefs regarding immunisation found in pregnant women, communities, and HCWs (e.g., failing to perceive pregnant women as at increased risk for disease, not believing that vaccination is a necessary preventative health measure, conspiracy theory thinking, eschewing medical providers in favour of "complementary" or "alternative" medical practices during pregnancy, and expressing concerns about safety of vaccines for pregnant women, fetuses, and infants);
- Lack of reporting and causality assessment of adverse events; and
- Ineffective communication of the risks by HCWs.
There are also barriers that impact HCWs' provision of vaccines during pregnancy, such as: lack of knowledge and education, misconceptions about the risk of the disease, concerns about the need for vaccination during pregnancy and vaccine safety and efficacy, lack of training on the technical and communication aspects, and misconceptions regarding their patients' preference for vaccination doing pregnancy.
Among the measures outlined in the article to address these and other barriers are: education tailored to the needs of physicians (including family physicians and obstetricians), HCW, and pregnant women (including peer-to-peer training/ mentoring); and IT support for timely monitoring of programme successes, challenges, and impact of adding immunisation in pregnancy to existing programmes.
Factors influencing vaccine hesitancy include: perceptions about the risk of the disease and disease severity; lack of recommendations by HCWs, government, and advisory bodies; lack of knowledge about vaccines during pregnancy; mistrust of vaccines; concerns about vaccine safety and effectiveness; fear of needles; lack of vaccines being offered by HCW, access to vaccination services, availability of vaccines and low antenatal care (ANC) participation; financial issues; lack of effective communication and dissemination of recommendations from HCWs and public health bodies; and societal factors like family influence, social norms, religion, and lack of decision-making autonomy/skills among pregnant women.
Measures to address vaccine hesitancy include:
- Education by HCWs (including nurses, midwives, and doctors);
- Strong HCW recommendations for vaccination, including verbal, face-to–face recommendations from a physician;
- Risk communication developed in collaboration with key stakeholders;
- Efforts to reach specific groups in the community (e.g., women's groups and community and religious leaders);
- Positive media coverage;
- High-quality obstetric care;
- Acceptable and affordable immunisation services offered through well-staffed clinics, pharmacies, churches/faith-based organisations, and other settings that are readily accessible by transportation, with convenient timings and absence of long queues; and
- Reminders and follow-up (including automated text message reminders about vaccination).
The article outlines various system-level considerations. For example, differences between the public health recommendations regarding the vaccine use in pregnant women and the absence of the indication on the vaccine label can lead to confusion in HCWs and pregnant women and result in lower compliance with national vaccination recommendations. To address the potential for misinterpretation of pregnancy-related language in vaccine inserts, new regulations instituted by the Federal Drug Administration (FDA) in the United States (US) require narrative descriptions (vs. letter ratings) of clinically relevant information on the risks of using the vaccine in pregnant and lactating women to be included on the label. As new data become available, the vaccine label needs to be updated. This may help to inform HCWs' counselling of pregnant women and communication of information on the benefits and risks of the vaccine use in pregnancy and lactation. Ultimately, having clarity regarding vaccine labeling related to pregnancy could help ensure that HCWs and pregnant women have a higher level of confidence in vaccines to be administered in pregnancy.
There are various ethical considerations of relevance in immunisation in pregnancy programmes, including:
- The principle of fair distribution of research benefits, which requires encouraging the participation of previously excluded groups, including pregnant women, in medical research and offering immunisation to pregnant women if the vaccines have been licensed or recommended for use in pregnancy in those countries;
- The principle of autonomy, which requires providing easily understandable, culturally sensitive, comprehensive information in the locally spoken language on the benefits and risks of immunisation so that pregnant women can make their own decision to get immunised;
- The need for active dialogue between pregnant women and HCWs in the community to understand pregnant women's beliefs about the necessity and safety of immunisation in pregnancy, which can contribute to the ethical justification and trustworthiness of the programme; and
- The need for adequate representation of women in decision-making bodies that influence national- and international-level policy decisions about immunisation in pregnancy programmes (including NITAGs, community advisory groups, scientific bodies, etc.).
Taking these factors into account, the paper concludes that "An investment in an immunization in pregnancy strategy would provide public health benefit by preventing infectious diseases in pregnant women and their infants and strengthening country antenatal care and health care delivery systems."
Vaccine Volume 37, Issue 25, 31 May 2019, Pages 3267-77. https://doi.org/10.1016/j.vaccine.2019.04.075
- Log in to post comments
